Applications and methods in reprocessing
A crucial component of the development process is to ensure that medical devices can be reprocessed with ease. Applicable standards, guidelines, and laws must therefore be observed and applied on the one hand, on the other hand the materials used must be able to withstand common reprocessing procedures.
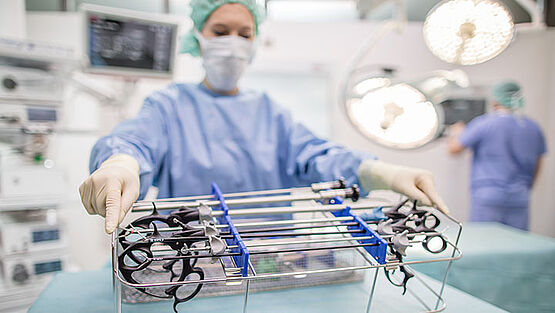
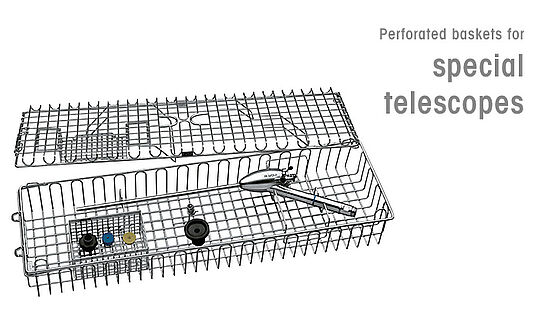
To ensure that processed devices are in perfect condition and to optimize handling in reprocessing (CSSD), as well as in surgery, Richard Wolf offers sophisticated storage systems and the corresponding accessories for reprocessing. Particularly where the automated reprocessing of telescopes is concerned, systems are required that ensure comprehensive protection against mechanical damage and that therefore do not limit the service life of these sensitive medical devices.
Professional reprocessing of Richard Wolf instruments and endoscopes requires extensive expertise on the part of the nursing staff. The increasing requirements in handling as well as the reprocessing of medical devices require an expert level of training in accordance with the applicable legislation and standards.

We differentiate between manual and automated cleaning.
As a mechanical aspect of manual cleaning, the suitable brushes play a crucial role.
Automated cleaning is followed by thermal disinfection, particularly in the case of medical devices sterilized with steam. It is essential that telescopes are fixed securely in the washer-disinfector. The corresponding equipment, such as telescope reprocessing baskets, are therefore required.
The instruction manual is an essential requirement for safeguarding the reprocessing of a medical device and must therefore always be taken into account.
After having used the instruments, clean them as soon as possible using water and additives (cleaning agents) that enhance the cleaning performance.
If instruments
- remain dry for a long time, coarse residues can dry onto the instrument and solidify, making them difficult to remove.
- are placed in liquids for a long time, they can become damaged. There is also a risk of damaging the seals on the instruments or rendering them ineffective.
The RIWO BOX container system from Richard Wolf provides a safe and complete system for manual cleaning, disinfection, and neutralization when processing contaminated instruments. Thanks to its practical design, simple and effective handling guarantees a safe and smooth work process in instrument reprocessing. The containers are available in various sizes.
Products for this application:

Manual disinfection techniques are primarily chemical processes using different agents. Ensure that the exposure time, concentration, and material compatibility with medical devices are all factors that are taken into account.
In automated reprocessing, the disinfection process follows the cleaning stage and can be a chemical, chemo-thermal, or thermal process, depending on the medical device and procedure.
The instruction manual is an essential requirement for safeguarding the reprocessing of a medical device and must therefore always be taken into account.
Manual disinfection
Observe the immersion time of the disinfectant as stipulated by the manufacturer and do not exceed this limit.
The RIWO BOX container system from Richard Wolf provides a safe and complete system for manual cleaning, disinfection, and neutralization when processing contaminated instruments. Thanks to its practical design, simple and effective handling guarantees a safe and smooth work process in instrument reprocessing. The containers are available in various sizes.
Automated reprocessing
In accordance with the international ISO 15883 series of standards, washer-disinfectors should only be used if they are expressly intended for cleaning and disinfecting endoscopic instruments.
A number of criteria must be observed:
- All instruments must be securely fixed to the inserts or baskets of the device.
- All inner and outer surfaces must be accessible by the reprocessing medium.
- Use special reprocessing baskets for telescopes.
- For optimum rinsing, instruments with channels / cavities must be adapted using special inserts with irrigation equipment or via direct Luer Lock connectors on the device.
- Remove stopcocks or place non-removable stopcocks in flow position.
- Open the jaw sections of the hand instruments / forceps.
If there is no sterilization after disinfection, the final irrigation should be performed using sterile water. The channels of flexible endoscopes can be flushed through with 70% alcohol (hygroscopic effect as a result of rapid evaporation) before drying for an improved drying effect before having air blown through – use a sterile cloth for drying on the outside.
Products for this application:

In contrast to disinfection, which is only intended to reduce the amount of microbes, sterilization is expected to remove all microbes capable of reproduction, including spores. In the field of minimally invasive procedures, sterility must be achieved by moist heat (steam-sterilization) or by low-temperature procedures with formaldehyde, ethylene oxide, or with hydrogen peroxide with and without plasma support.
Sterilization baskets with outer packaging
Richard Wolf supplies an extensive range of basket systems for sterilization procedures involving steam, plasma, ethylene oxide, and formaldehyde. The various baskets must be individually equipped for the relevant specialist areas.
The standard sizes of the basket system can be fitted with sterile packaging or can also be sterilized in sterilization containers, such as those provided by Martin, Aesculap, or Wagner, for example.
Products for this application

The reprocessing and care of medical devices has a significant effect on their service lives.
The following advice is intended to complement the detailed reprocessing information provided in the product-related instruction manuals.
Use of cleaning agents and disinfectants
Richard Wolf recommends only using products that we have tested for material compatibility. If you use other cleaning agents or disinfectants, the material compatibility of the processing agent with our endoscopes should be confirmed by the manufacturer.
Approved chemicals for processing
The material compatibility of the following chemicals has been approved by Richard Wolf for reprocessing rigid endoscopes, flexible endoscopes, and instruments.
The material compatibility with Richard Wolf products only relates to the use of the specific reprocessing chemicals listed. Due to the large number of chemicals on the market, possible interaction with other products cannot be taken into account. The manufacturer’s instructions on exposure time, concentration, and use must be followed. It must also be ensured that no residues remain on the instruments.
Determining the concentration of solutions
Damage to surfaces (made of medical steel, stainless steel, plastic, etc.) may be caused by highly concentrated solutions, which could substantially reduce the service life of the instruments. This could also increase the costs for cleaning agents and disinfectants.
Specified immersion times
Please observe the specified immersion times for cleaning agents and disinfectants, which must not be exceeded under any circumstances.
Switching between manual and automated reprocessing
Frequent switching between reprocessing procedures exerts an increased strain on the materials and should be avoided. If you change between manual and automated reprocessing, you should ensure that the reprocessing agents used are compatible.
Recommendations on the reprocessing of instruments in hospitals are summarized by the AKI (Instrument Reprocessing Working Group) in the brochure “Instrument Reprocessing – Reprocessing of Instruments to Retain Value”.